PROMPPT Updates

Intervention development publication: Using an online discussion forum to hear the patient voice
23rd JULY 2024
Published in the British Journal of Pain, we describe how we used a bespoke online discussion forum to hear the views, beliefs, and experiences from people living with persistent pain. Our online discussion forum was called the Q-PROMPPT blog and in this paper we provide recommendations for researchers who wish to use an online discussion forum as an innovative method for qualitative studies during intervention development.

British Pain Society – 57th Annual Scientific Meeting, Nottingham
4th- 6th JUNE 2024
PROMPPT’s PI, Dr Julie Ashworth, represented the PROMPPT team at the 57th annual scientific meeting of the British Pain Society hosted in Nottingham, UK. Julie presented a conference poster reporting key findings from the internal pilot of the randomised controlled trial.

Intervention development publication: Designing a primary care pharmacist-led review
17th APRIL 2024
In the latest publication from PROMPPT, we identify facilitators of and barriers to patients reducing opioids in the context of a pharmacist-led review in primary care. Understanding what makes it easier for patients to talk about their pain medicines and consider making a change is important for designing a review which is relevant and acceptable to patients. This study is published in the British Journal of General Practice Open.

PROMPPT main trial recruitment target reached
30TH JANUARY 2024
We are pleased to report that the PROMPPT cluster randomised controlled trial has reached its recruitment target. The trial recruited its first participant in May 2022 and has now recruited over 900 participants from 38 GP practices across England. We are grateful to all of the participating practices and patients who have made this possible and would like to thank West Midlands CRN and Keele University’s CTU for their commitment and support for this study.

Intervention development publication: Acceptability of a PROMPPT review
19TH DECEMBER 2023
The first peer reviewed journal publication from PROMPPT has been published in the British Journal of Pain. The article, led by Dr Nicola Cornwall, explores the acceptability of a proposed PROMPPT review for patients and clinical pharmacists.

British Pain Society – 56th Annual Scientific Meeting, Glasgow
9TH – 11TH MAY 2023
Members of the PROMPPT team attended the 56th annual scientific meeting of the British Pain Society hosted in Glasgow, UK. The team were thrilled to share with conference delegates work from the past 4 years and discuss PROMPPT going forwards…..
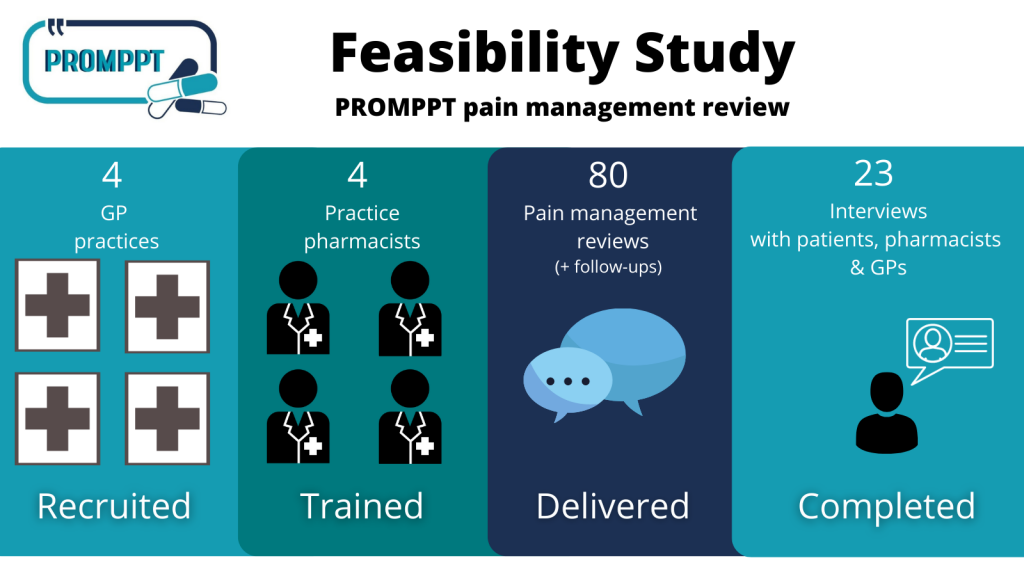
Feasibility Study: Summary
2020-2021 PROMPPT conducted a Feasibility Study to test whether a full randomised controlled trial was possible to test the effectiveness of the PROMPPT review….

British Pain Society – 55th Annual Scientific Meeting, London
13th – 15th June 2022
We were delighted that that an abstract with the title, Stakeholder involvement in the development of a new proactive clinical review of patients prescribed opioid medicines long-term for persistent pain, was selected for a poster presentation at the 55th…

Patient & public involvement (PPI) meeting
28th April 2022
We met with our patient advisors from the Keele Research Users Group to update them on work we have been doing revising the PROMPPT intervention (the pain review and the pharmacist training package) ahead of the main PROMPPT trial …
Seasons Greetings from PROMPPT
21st December 2020

There’s no doubt that 2020 has been a challenging year for all. Since the onset of the pandemic we’ve all had to learn to do things differently.
We reflect on how we have adapted the PROMPPT programme in our latest update.
Whilst Christmas may be a little different this year we send our very best wishes for the festive season and 2021.
PROMPPT Feasibility study gets the green light
18th November 2020
The PROMPPT feasibility study has been granted the green light from Keele University’s Clinical Trials Unit. The green light process is the final step of study set up before recruitment can begin.
What is a feasibility study?
A feasibility study is conducted to find out whether a proposed service is appropriate for further research testing. The PROMPPT feasibility study will determine whether the PROMPPT pain management review should proceed to the next phase of the research programme.

Invitations to potential participants for the PROMPPT feasibility study are now being sent out. Participants who agree to take part will help test the acceptability and feasibility of the new PROMPPT pain management review. The review will be delivered by clinical pharmacists in primary care for people living with persistent pain and take regular opioid medicines (>6 months).
The PROMPPT pain management review is underpinned by the current evidence-base and a year-long piece of qualitative research, called Q-PROMPPT, collaborating with patients, public, healthcare professionals and key stakeholders. We are excited to begin the feasibility study and test the review across 4 GP practices in the East and West Midlands to critically assess how acceptable the review is to patients, pharmacists and GPs.
What makes a pain management review acceptable?
Acceptability is an important component of success. Broadly, acceptability of the PROMPPT pain management review will depend on its perceived value, effectiveness, appropriateness, and accessibility to patients, clinical pharmacists, and GPs.
The findings from feasibility testing will help improve the review before the next project phase, a large randomized control trial.
A bespoke training package has been developed for the clinical pharmacists delivering the review. In light of the current pandemic, all of the training has gone online, and pharmacists will be supported throughout by the research team and clinical champions.
Building shared experience into the PROMPPT review
30th September 2020
Drawing on the rich information shared in our interviews, focus groups, Q-PROMPPT Blog and wider PROMPPT community (PPI and stakeholder groups) we are developing the PROMPPT pain review and associated clinical pharmacist training package.
“I think the patient with life experience is one of the best tools a clinician has in his bag! It’s not a GP telling them you must stop your meds, or a physio telling you to exercise. But a real person who has just been through everything”
Q-PROMPPT Blog participant (male)
Members of our PPI group and Q-PROMPPT participants told us it’s important to hear stories from others who are living with pain and have experiences of taking regular opioids.
“recovery stories are few and far between so hearing from anyone is beautiful”
Q-PROMPPT Blog participant (female)
As part of the PROMPPT programme we are pleased to support the development of Live Well With Pain‘s latest video of Louise Trewern’s inspirational story of living with pain. We are excited to share Louise’s video below. A link to Louise’s video will also be shared with everyone who attends a PROMPPT review as part of the patient resources.
NICE guidelines for chronic pain management
23rd September 2020
The draft guidance published by NICE on chronic pain management was published at the beginning of August. Our qualitative research (Q-PROMPPT) has informed us that it is important for patients to have a collaborative and supportive relationship with the health care professional they consult with (in PROMPPT this would be a clinical pharmacist) and this message was consistent with that in the draft guidance.
The research team has responded with our thoughts on the draft guidance along with other clinicians and research staff from the Medical School at Keele University and we expect the final guidance to be published in early 2021.
To top it off in the same week our principal investigator, Dr Julie Ashworth, was invited to comment on the guidance published on our local radio station (BBC Radio Stoke) in her role as honorary consultant in pain medicine with the Midlands Partnership Foundation NHS Trust. In a year when there has not been much to celebrate, a little stardom for Julie and the team came as a much needed morale boost.
A brief update from the PROMPPT team
16th September 2020

Almost six months has passed since the UK was put into lockdown due to COVID-19 and the PROMPPT team having been working from home all this time.
Despite the challenges of lockdown, we’ve made some real progress with the next phase of the PROMPPT research programme to test the feasibility of the PROMPPT pain management review and training package:
- Our qualitative research team has been able to continue working remotely analysing the data from the Q-PROMPPT blog, interviews, focus groups and in-practice testing.
- Our intervention and training development team have been developing the (now 100% online) training for Clinical Pharmacists.
- With the support of the Keele Clinical Trials Unit, the protocol for the feasibility study was approved from the designated Research Ethics Committee and Health Research Authority, registered with ISRCTN and given the go ahead by the sponsor’s office in Keele University.
Right now, we’re recruiting four GP practices in the West and East Midlands to take part in the feasibility study. We will be training clinical pharmacists so that they can deliver the PROMPPT pain management reviews in real time.
Thank you to everyone who has been involved in PROMPPT and supporting our work. We look forward to sharing more with you in the future.
PROMPPT is 1!
2nd March 2020

The PROMPPT programme officially started one year ago this month. We celebrate the last 12 months with a PROMPPT update. In our latest newsletter you can read about:
- How the PROMPPT intervention was designed
- PROMPPT’s first year in numbers
- Update from the Q-PROMPPT Blog
- Next steps…
Third PROMPPT Stakeholder workshop
5th February 2020

On the evening of January 30th 2020, 14 stakeholders from primary care (GPs, practice nurses, practice managers, clinical pharmacists), health psychology, addiction and community pain services, and patient representatives met for a third and final time in the intervention development phase of the PROMPPT programme. In the meeting stakeholders were given an overview of the work that has been completed since their last meeting in September 2019. Dr Sarah Harrisson shared developments of the pain review to be delivered by clinical pharmacists and supporting materials. Dr Nicola Cornwall also provided an update of Q-PROMPPT qualitative study findings taking into account information from the 4 different elements of the study:
- GP, clinical pharmacist, and patient interviews
- Clinical pharmacist focus groups
- Online research forum (Q-PROMPPT Blog)
- In-practice testing of a prototype pain review
Discussions ensued on key areas for the on-going iterative development of the intervention. We would like to express our gratitude to all our stakeholders and the expertise they have shared for the PROMPPT programme. We hope you have enjoyed being part of the PROMPPT journey and look forward to the next phase of the project.
PROMPPT team to present development work at a prestigious research and science conference
23rd January 2020
This week the research team found out that an abstract with the title, Development of a primary care review for patients prescribed opioid medicines for persistent pain, led by clinical pharmacists, has been accepted for an oral presentation at the PHE Public Health Research and Science Conference 2020, taking place at Manchester University on Tuesday 31 March and Wednesday 1 April. This will be the first opportunity for the team to share some of the findings of the development work undertaken during the first phase of the research programme.
Q-PROMPPT Blog is now closed
18th December 2019
After 11 weeks of being online to participants, the Q-PROMPPT Blog is now closed. The Q-PROMPPT study team would like to thank everyone who participated in the blog. We are very grateful to you for taking the time to share your experiences and views. We are in the process of analysing all the comments posted to help us develop a new way for clinical pharmacists working in GP surgeries to improve the care for people taking regular opioids for long-term pain. We will test how well this works in future studies.
If you would like to keep updated about the PROMPPT research programme and the findings of the Q-PROMPPT research blog, please visit the PROMPPT website where we will post regular updates.
We will also publish results, as they become available on the Keele University Research Institute for Primary Care & Health Sciences website in the new year.


