PROMPPT is for…
Primary care practices
PROMPPT is a 5-year research programme and aims to develop and test a new pain review for people who are prescribed opioids long-term for persistent pain . The pain review will be delivered within a patient’s GP practice, by the practice pharmacist.
The PROMPPT trial will explore whether proactively inviting patients to a practice pharmacist-delivered pain review reduces opioid use without worsening pain or pain-related interference with quality of life
How are GP practices involved in PROMPPT?
GP practices and healthcare professionals from primary care teams have been integral to every phase of the PROMPPT research programme.
Work phase 1

- GPs, practice pharmacists and patients were interviewed about current healthcare of persistent pain patients and how it could be better supported in the context of primary care
- A mixed stakeholder group including GPs, clinical psychologists, physiotherapists, addiction specialists, practice pharmacists, pain patients, practice nurses and GP practice managers helped design the proposed pain review and advised on delivery prior to initial testing of a prototype
Initial testing and refinement of the pain review was conducted with help from three West Midlands GP practices and their pharmacists.
Work phase 2
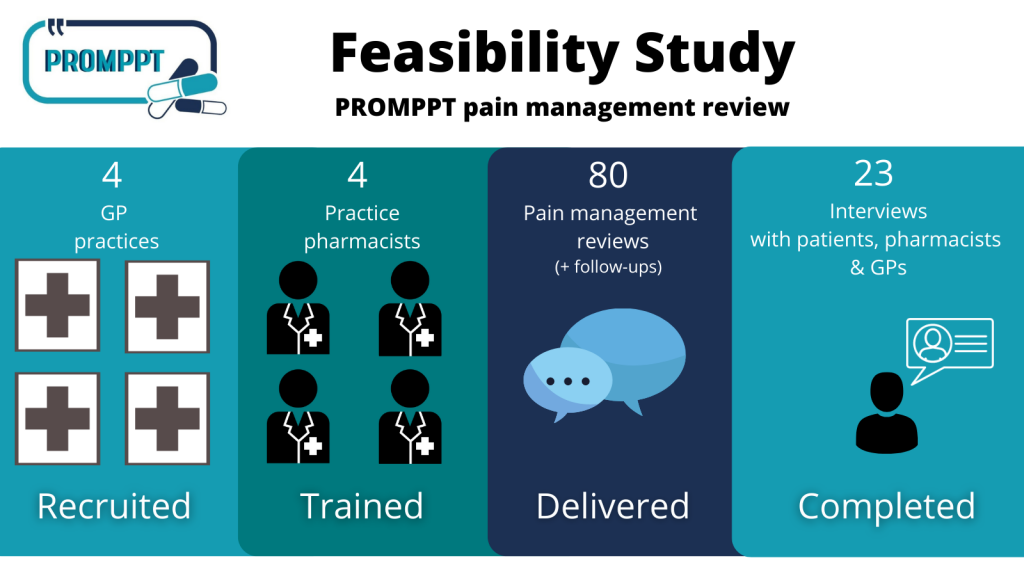
Four GP practices and their pharmacists took part in a feasibility study to test:
- whether patients invited to a pain review would book and attend an appointment
- whether the training package was acceptable to practice pharmacists and enabled them to deliver the review
- whether the pan review was acceptable to GPs, practice pharmacists, and patients
Practice pharmacists delivered 80 initial pain reviews.
Questionnaire and interview data supported the feasibility of the new pain review and informed refinement of the review and training package ready for testing in a randomised controlled trial.
Work phase 3

GP practices from the West Midlands, East Midlands, Wessex and Thames Valley regions have been recruited to take part in a randomised controlled trial.
The trial in the process of testing whether:
- patients who attend a pain review (and follow-ups) are more likely to reduce their opioid use (without increasing pain/pain-related interference) compared with their usual care
- providing practice pharmacist-led pain reviews is cost-effective compared to usual GP practice arrangements for review of patients on long-term opioids
Recruitment for the PROMPPT randomised controlled trial is now closed.




